+91 79 48918418
B-1213, Empire Business Hub Nr. Water Tank, Science City Road, Sola, Ahmedabad – 380060
» Research & Development
Research & Development
Accelius Global has a state-of-art Research and Development Centre (R&D) at towards developing new generic products, improving existing products as well as drug delivery systems and expanding product applications.
R&D Centre have highly experienced team of scientists having post graduate and doctorate degrees in the field of pharmacy and chemistry.
The Galenical laboratory is about 4200 sqft in area.
The R & D Centre is competent to handle entire activities from Product Identification, Lab Scale Development to full scale Batch Manufacturing.
The Centre supports design and register formulations in its various markets, detailed study and documentation is generated with the help of regulatory department for various country specific registrations for ROW Countries.
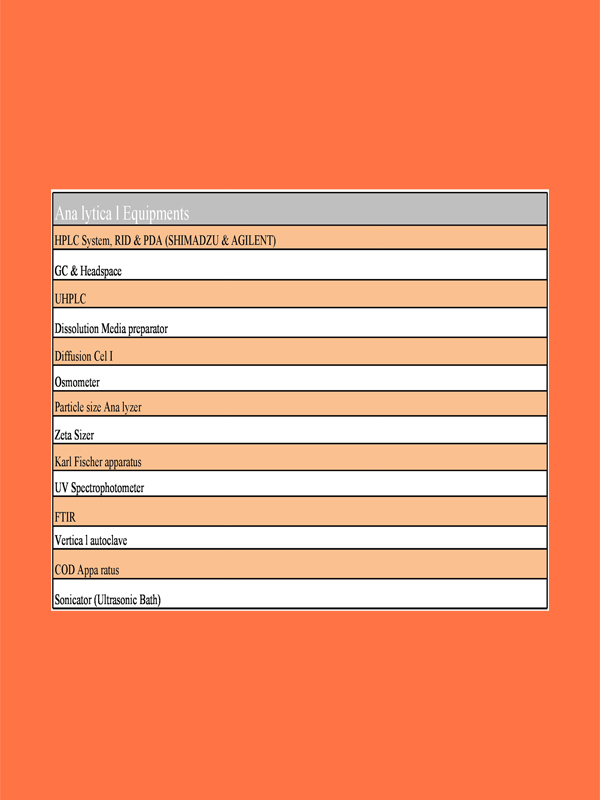
Our R & D capabilities include
- Formulation & Research Development
- Dedicated Regulatory Team for Global Filings
- Dossier Development
- Process Optimization
- Analytical Development
- Conducting Stability Studies
Our strategy focuses on
- Extended or delayed release products and New Combination products.
- Solubility & Permeability Enhancement Technologies
- Alternative Pharmaceutical Dosage Forms
- Conducting Clinical and Bio-Equivalence studies for obtaining regulatory approvals for new products and services.
- Wide range of products in Pipeline at Various Stages
- Expertise in developing generic (Oral Tablets & Capsules)
- More than 11% revenue invested in R & D
International Regulatory Strategy
Regulatory Strategy is the key to optimizing the development resources for global development of finished products.
- Assess the global regulatory landscape based on each local regulatory and market needs ( ACTD or CTD).
- Formulate CMC and BE related queries effectively on time.
Our team is engaged in day-to-day development of dossiers interacting with all the manufacturing sites to keep updating them for regular regulatory developments and features in the international arena.
Our DRA is constantly and continuously engaged in product registrations in various markets (domestic and emerging) across continents. The core responsibility of our DRA Team is to register products in the international and domestic markets, in order to gain access for such products in shortest possible time.
Disclaimer : Products protected by valid patents are not offered for sale in countries where the sale of such products constitutes a patent infringement and its liability is at buyer’s risk. Products currently covered by valid US patents are offered for R&D use in accordance with 35 USC 271 (e)+A13(1).
© 2018 Accelius Global. All Rights Reserved. Design developed by Shah Infotech & Inner Engineering Pvt.Ltd.